Acid rain is any form of precipitation that is uncommonly acidic and extremely corrosive in nature. That means it has elevated levels of hydrogen ions (low pH).
Acid rain is not only rain. It can take many different forms, such as fog, snow, hail, dew and mist.
Check out the below infographic, it may help you to understand how acid rain is formed and how it affects our environment.
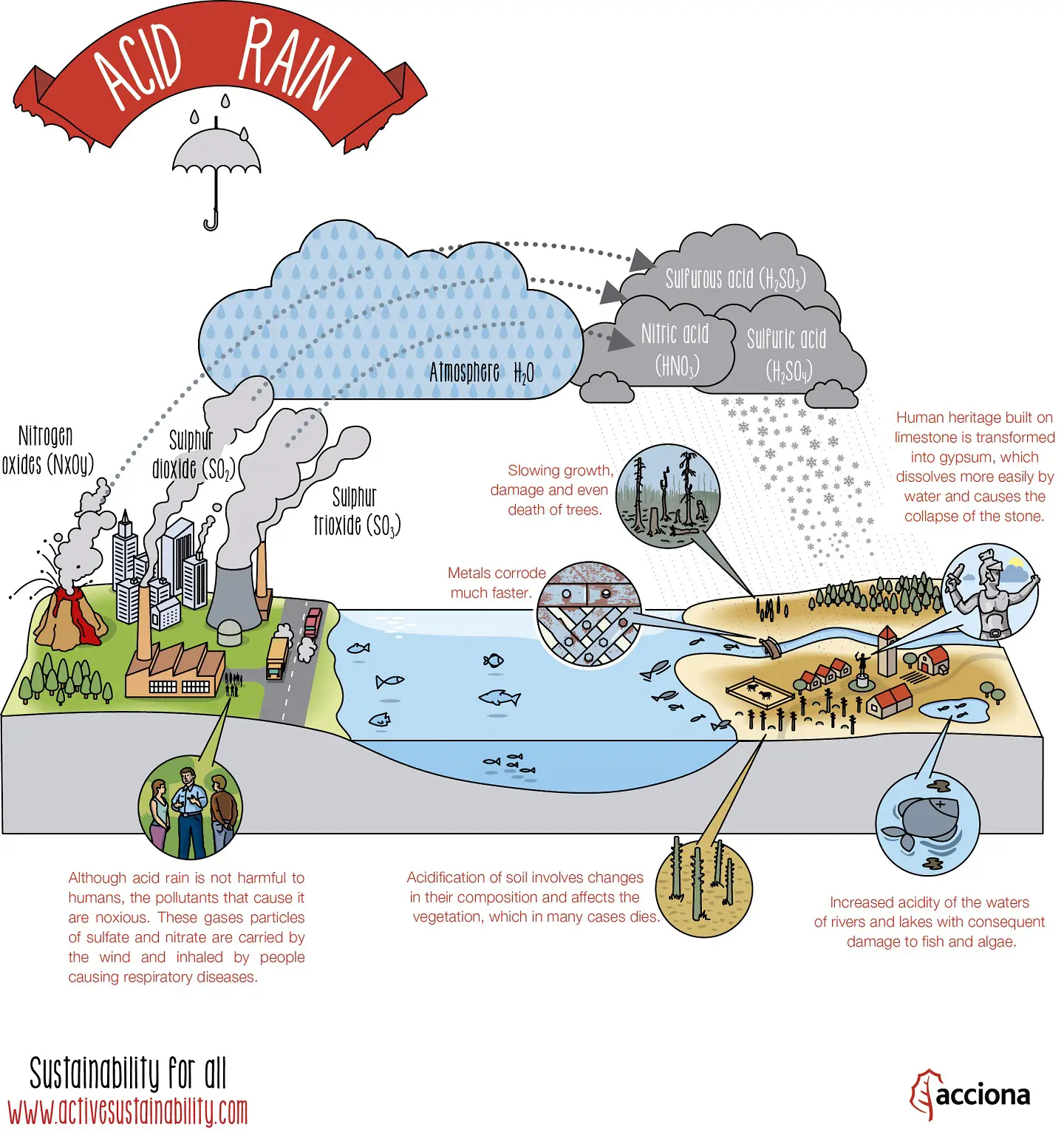
Table of Contents
How is Acid Rain Formed?
Acid rain is caused by both, natural and human-made means.
Decomposing vegetation and erupting volcanoes release some chemicals that can cause acid rain naturally. However, human beings have been the major cause for acid rain since the industrial revolution.
Sulfur dioxide (SO2) and nitrogen oxide (NOx) are released into the atmosphere when humans burn fossil fuels. These air pollutants respond with water (H2O), oxygen (O2) and various other substances to form floating sulfuric (H2SO4) and nitric acid (HNO₃)
The acid compounds can be spread hundreds of miles throughout the atmosphere by winds.
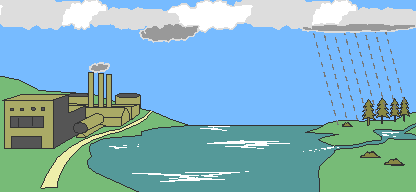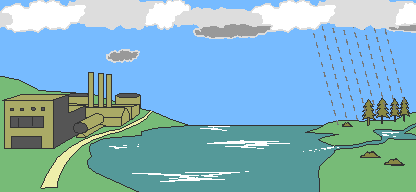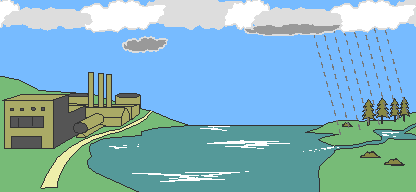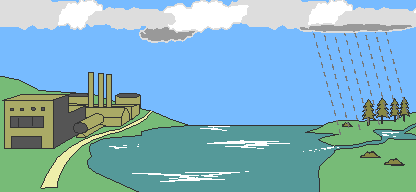
Eventually, the atmospheric acids reach the earth in the form of rain (or various other forms). It then flows over the surface in runoff water, sinks into the water table (the level below, in which the ground is saturated with water) and enters water systems.
The below graphic shows you how our natural water cycle works. Visualize the water being contaminated with acid. When you do this, it sheds new light on just how far acid rain can penetrate our environment.
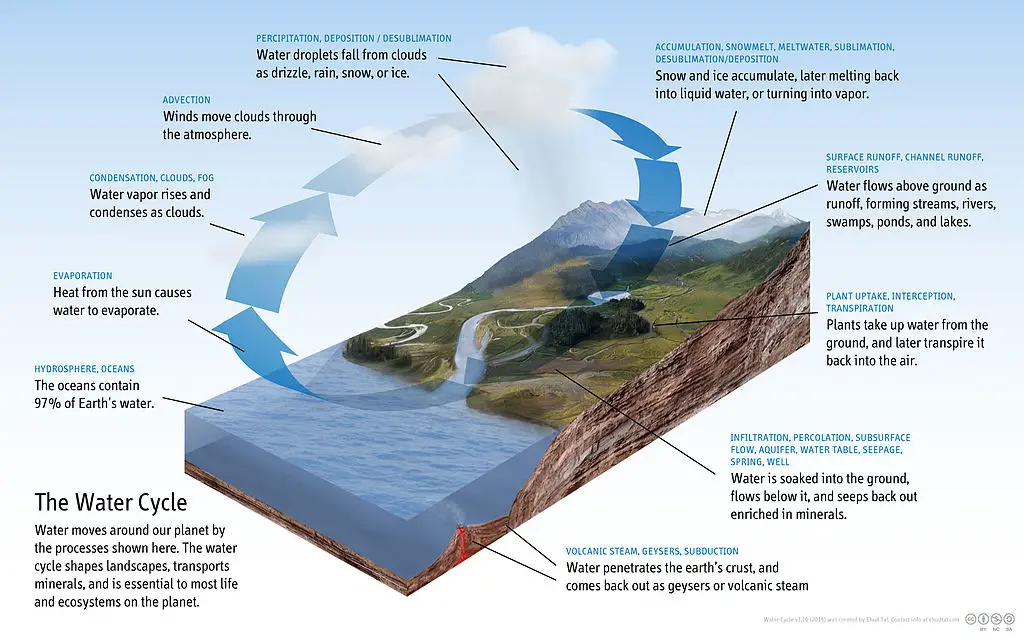
How Harmful is Acid Rain?
Effect on Humans
Physical acid rain itself is not harmful to humans. Thus, rain that comes into contact with your skin does not pose any health risks. However, all the gases that form this rain (nitrogen oxides, sulfur dioxide and sulfur trioxide), are harmful. If the small particles within the gas are inhaled, namely sulfate and nitrate, respiratory disease can occur.
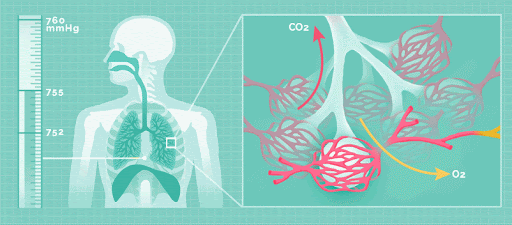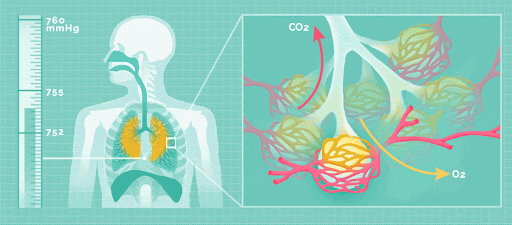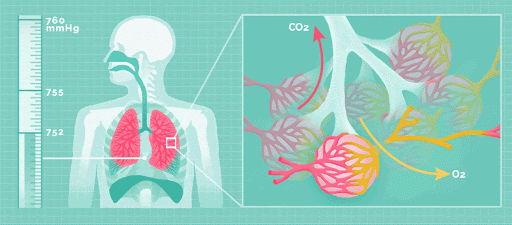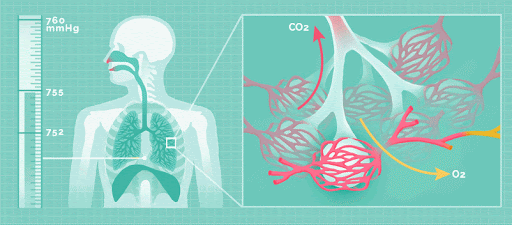
Effect on the Environment
Acid rain can devastate forests (the acid wipes out nitrogen-fixing microorganisms and destroys leaves and branches on contact) and soil, as well as poison freshwater rivers and lakes.
Read: Reforestation, Afforestation and Deforestation Explained
Heritage sites such as buildings and monuments can be severely damaged by acid deposition. Chemicals within the acid tend to react negatively towards calcium carbonate stone. As a result, marble and limestone statues and monuments are eroded.
Effect on Animals
There are some species of plant and animals that are able to tolerate acid rain and its effects. Others, however, are rather acid sensitive and will die off, as the pH levels decline.
Generally, most of the younger animals are more sensitive to acidic environmental conditions.
If pH levels reach 5, most fish eggs cannot hatch. Some adult fish even die as pH levels decrease. Even if a certain species of fish or animal can tolerate acidic water, the animals and plants the hardy species eats cannot. Thus, some acidic lakes around the world have absolutely no fish or animals. Ghost lakes…
For example, some frogs have a pH of around 4, but the insects they feed on (mayflies) have a more sensitive pH of 5.5. Thus, the frog has no food to eat and ultimately starves to death.
Read: 21 Animals in Danger of Extinction
Countries that Produce the most Acid Rain
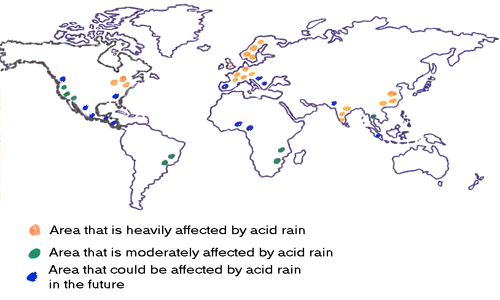
The Black Triangle
The black triangle is an area that received large amounts of acid rain during the 1970s. The area covered parts of Czech Republic, Germany, and Poland.
Within parts of the black triangle, entire forest systems were destroyed. Even railroad tracks began eroding due to the highly acidic precipitation.
In 1979, the Geneva Convention created strict regulations to control the emissions of coal burning factories. Since the intervention, acid deposition was significantly reduced in the region.
Germany

Out of all the European countries, Germany produces the most acid rain. The country is a major steel producer and coal consumer.
The most valuable place acid rain has affected in Germany is the vast black forest, which covers a total area of 6900km².
India

India produces exceptionally large amounts of iron and steel. However, the bigger problem is the country’s reliance on coal and fossil fuel.
The amount of motor vehicles operating around the country (the 3rd most in the world) is increasing India’s production of acid rain.
One of India’s worst polluting cities is Chandrapur. 12 coal-fired power stations are located here. 70% of the children living in this city develop asthma at an early age.
United States of America

The United States of America have the most registered cars in the entire world, around 279 million. Also, the country has the most nuclear power plants and is ranked 2nd in the world for the most coal consumption.
The USA is also responsible for producing most of the acid deposition on the South East of Canada.
China

China is responsible for approximately 28% of the world’s CO2 emissions.
Rapid industrialization has caused China to be the world’s biggest iron and steel producer. They are also the number one country when it comes to coal consumption.
What are the solutions to acid rain?
The only way to prevent acid rain from destroying the environment and harming animals is to slow down the release of chemicals that cause it.
What this means is simple, yet complex. Countries need to burn fewer fossil fuels and set air quality standards.
For an acid free atmosphere, countries need to switch to renewable energy sources and reduce their overall use of fossil fuels in the industrial and automotive sector.
Read: Best Off Grid Solar Panels
Still not sure what acid rain is?
Check out the video below.

James Browning
Wednesday 20th of May 2020
Another highly informative and educational narrative. Human beings should strive to learn something new every single day, and your blogs provide a fantastic forum to do just that. Keep up the sterling work.